FDA's shortcomings: A list
Or how We the People can regain control of the FDA and improve our nation's health
I just attended a Public Health Integrity Committee meeting today. This group was appointed by the State Surgeon General of Florida, Joe Ladapo, under Ron DeSantis, and includes Jay Bhattacharya, Martin Kulldorff, Joseph Fraiman, Christine Stabell Benn, Bret Weinstein, Steve Templeton, and me. We meet regularly to provide an alternative voice and to the CDC and FDA. I also like to think of us as helping provide an avenue for the public to question and critique these agencies. This is why I am writing a Substack about what happened today - because I want your input.
Today we met about the FDA, specifically its recent shortcomings. Edit: Here is the press release from the meeting and few quotes from the members
Background
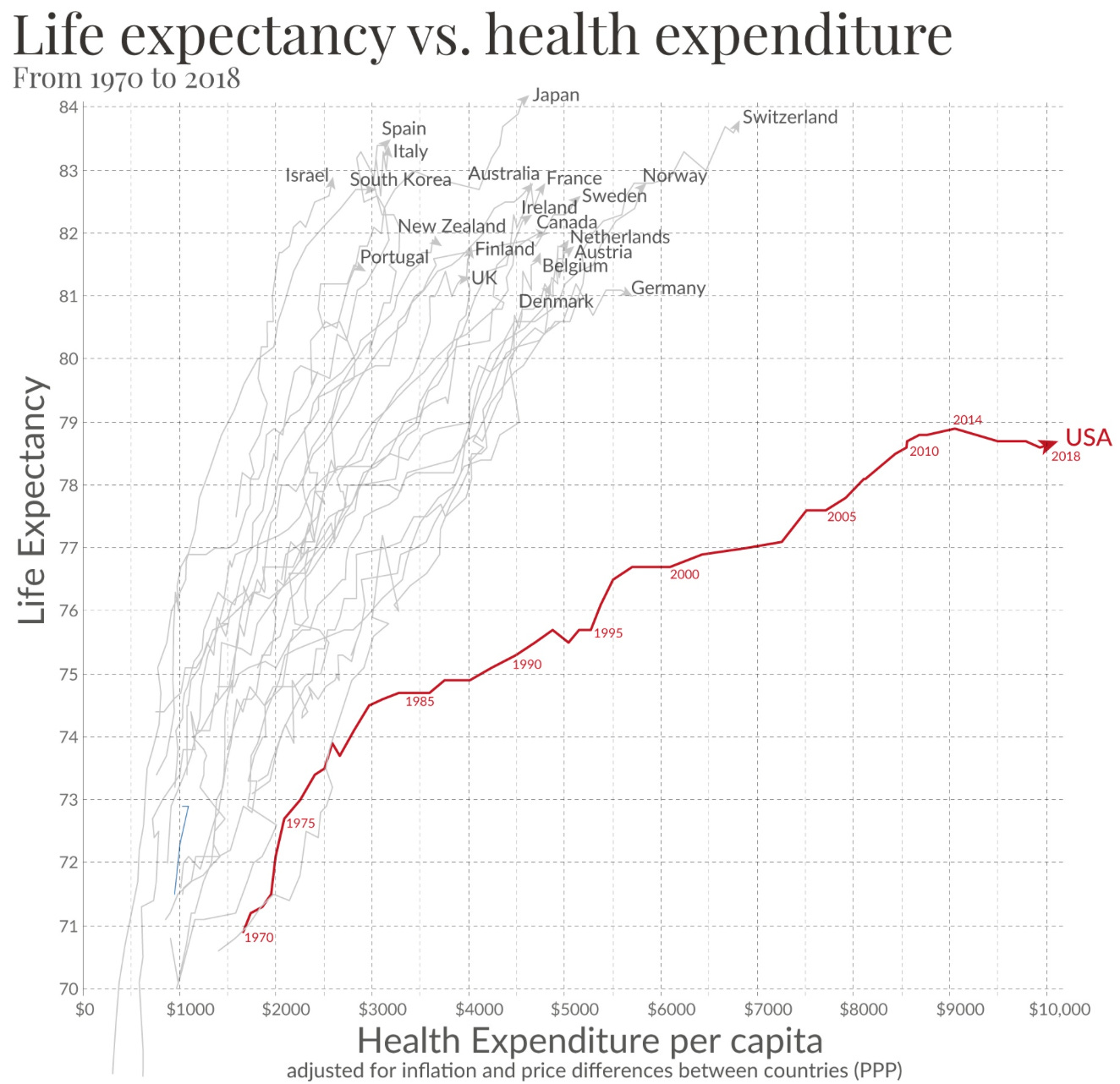
I want to start with some big picture remarks. The US is spending more and more healthcare $ per capita and not making clear gains in terms of health.
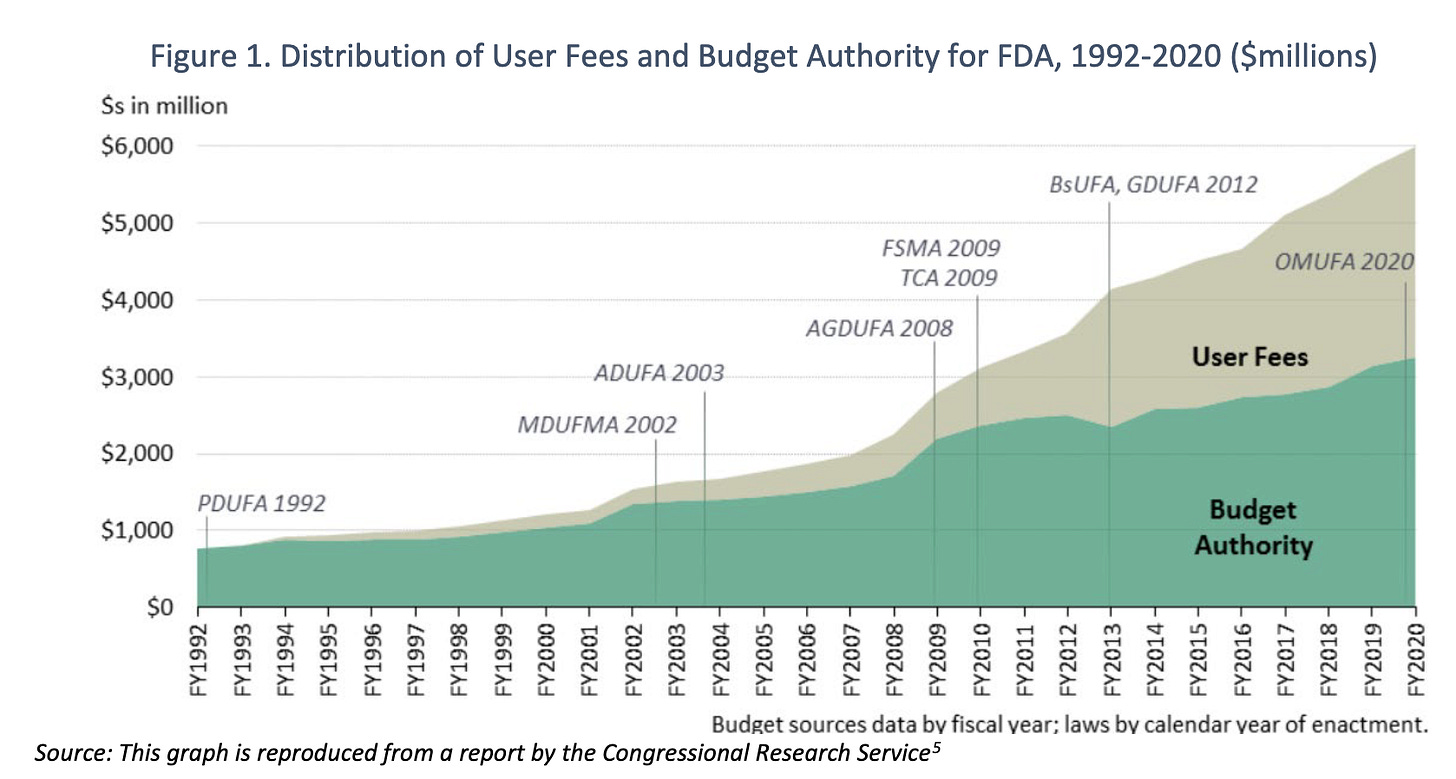
The FDA’s budget is rapidly increasing and this is in a large part funded by “user fees.”
User fees from industry made up $2.9 billion (or 47%) of the FDA’s total $6.2 billion budget in 2022. (And actually all industry funds in total [including but not limited to user fees] make up 65% of the FDA’s budget, according to an investigation in the BMJ by Maryanne Demasi). User fees did not exist before 1992 when the prescription drug user fee act was passed, allowing the FDA to collect fees from drug manufacturers to fund (and importantly speed up) the new drug approval process. These fees sound like they could theoretically be a good thing--if they bring more safe, effective drugs and other products to the market in an expedited way. But instead what we have seen is regulatory corners being cut and the post marketing surveillance/monitoring not being able to keep up with everything that has been approved.
It’s also becoming increasingly clear the FDA is either not capable or not willing to respond to public inquiries or keep up with the data coming into VAERS and other safety monitoring systems (see below). They are also relying to an increasing extent on unvalidated surrogate endpoints for their trials. These are endpoints that may or may not translate into true health benefits (think having endpoints of increased antibody levels instead of decreased levels of severe disease; or smaller tumor rather than longer survival).
What has become abundantly clear over the last 30 years, and really brought to the fore during the opioid epidemic and then again the Covid years is that our health is not improving per health care $ spent. National trust in our public health agencies is diminishing, and our insurance premiums are increasing, while our life expectancy, unlike other high income nations, is not. But what we do have is more .... products and drugs. Here is a photo from the meeting today where Dr. Linda Simoni-Wastila gave us a brief history of user fees and the problems they have brought to the FDA.
What exactly has gone wrong with the FDA?
It’s far from just these “user fees” creating problems within the FDA. There is the revolving door, the conflicts of our major medical journals created by their funding (largely pharma advertising and reprints; see the linked great study by my colleague from Cochrane Denmark/SDU Andreas Lundh), the unwillingness of our media and academic institutions to investigate the FDA because of their own funding conflicts. So what can our little Public Health Integrity Committee do? What can We the People & readers of this Substack do?
One potential answer is to increase public awareness of the problems within our institutions and embarrass them into accountability and change (also alert politicians who have leverage).
To that end, I am drafting a list of specific ways and concrete examples of how the FDA has been a disappointment in recent years. I hope you will help me add to this list and/or speak up if you disagree with anything I mention.
Post marketing surveillance
Post marketing surveillance and Phase IV trials is realistically how we identify rarer side effects and safety issues as well as evidence of diminished real-life efficacy. While Nordic countries have designed cluster RCTs to determine the safety and efficacy of vaccines during the vaccine rollout, the US is dropping the ball on monitoring safety and efficacy post-approval over and over.
Dr. Simoni-Wastila noted there were 7 post-marketing studies related to the COVID-19 vaccines that are overdue/delayed/released from completion without explanations being given to the public. I have been working on creating a list with Jessica Adams. (It’s in and of itself frustrating that it’s so difficult to find which FDA requested covid vaccine safety studies are delayed/not going to be done after all).
Comirnaty subclinical myocarditis in 16-30 year olds Study C4591031, due a year ago and still no results
ii. Spikevax occurrence of myo and pericarditis, no results available yet. Due 6 months ago
iii. Moderna Spikevax booster subclinical myocarditis. Due 6 months ago. No results available yet.
iv. Pfizer released from evaluating immunogenicity and safety of lower dose levels. Why?
v. Moderna released from pregnancy outcome study- why?
vi. Per Clinical Trials, no subjects yet enrolled
I’m only getting 6 but I’m hoping you all can let me know what I am missing.
Safety (VAERS)
In addition to CDC not reacting in a timely way to VAERS reports, as we demonstrated when our research group uploaded a preprint in August of 2021 ahead of the CDC, with more accurate numbers of post-vaccination myocarditis in adolescents than they had been giving to the public, our health officials also hold onto VAERS data until they feel the time is right to release them to the public. This is still happening with the reports of myocarditis following the bivalent covid vaccines, as Zack Steiber describes here
A recent article in the BMJ by Jennifer Block outlines the almost unbelievable frustrations of physicians reporting child deaths to VAERS. One was Patrick Whelan, MD, PhD from UCLA who has been unable to update the status of a child’s cardiac arrests after covid vaccination to “death” rather than “hospitalization” and another Dr. Gill has been unable to learn why a child death that was found by autopsy to have been due to the vaccine was not reported as such in VAERS or acknowledged to be due to the vaccine by the CDC.
The fact that 22% of VAERS reports were never given a permanent VAERS ID number is something the FDA and CDC should promptly address.
Revolving Door
Peter Doshi writes a recent investigation in BMJ about how two FDA regulators, Doran Fink and Jaya Gaswami, who had just been working on Moderna products took jobs at Moderna two months or less after leaving the FDA. This is, of course, nothing new, but it’s important for people to realize it is ongoing.
Approvals are based on trials that are too small.
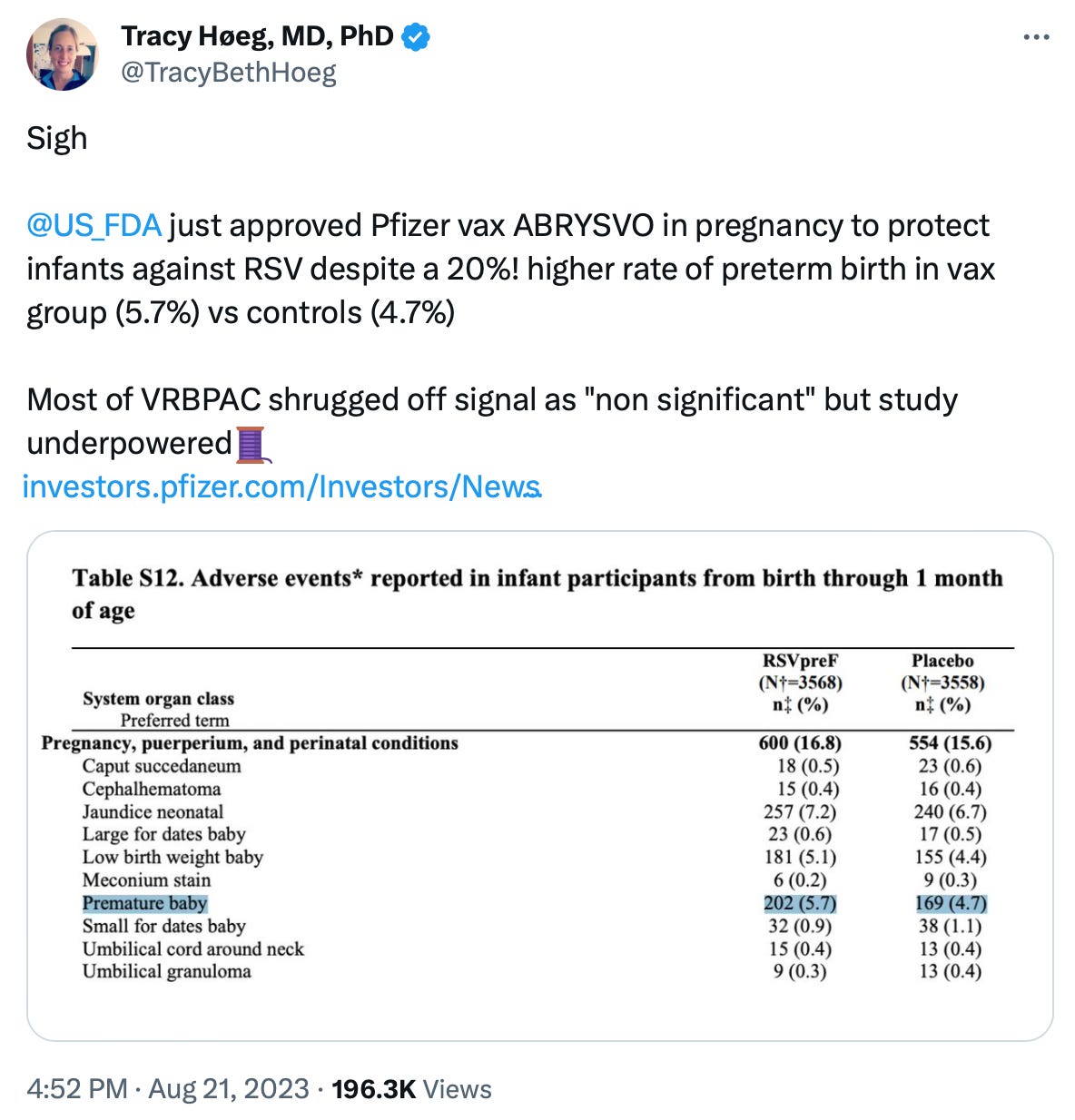
We certainly saw this with the child COVID-19 vaccine approvals, but also more recently with the RSV vaccine for pregnant women when the study was underpowered to determine if the >20% increased incidence of preterm birth in the Pfizer vaccine group was significant.
Rather than the FDA asking Pfizer to increase the size of the trial by just 1400 people per arm, FDA just approved it and said they would, you got it, “rely on post marketing surveillance” (see above) to determine if this 20+% increase was real. Oof.
Very slow process of removing drugs from the market that are not just inefficacious but dangerous.
This was seen with the drug Makena given to prevent preterm birth (Dr. Adam Urato is an amazing follow on this topic). It was approved in 2003 based on a very small randomized trial (published in NEJM, where else?) where the randomization appeared to have failed giving misleading results. Subsequently multiple studies not only found the drug failed to prevent preterm birth but increased the risk of stillbirth and miscarriage. It was given for 20 years before being withdrawn and I suspect we are poised to see a similar story with the RSV vaccine for pregnant women.
But this gets me to the very important point that the conflicted medical journals influence the conflicted FDA and CDC so they simply reinforce each other and it has become extremely difficult for the public and non-conflicted physicians and scientists to counter or even see through to begin with. As I mentioned above, scientific journals just LOVE positive drug trials because they make a ton of. money (I’m working on a study on this topic right now so I’m extremely interested to connect with people with more information specifically on the funding of the NEJM)
Lack of Transparency.
If the FDA is having trouble keeping up with post-marketing surveillance studies and safety data, they should be happy to make their raw data available to the public. This way independent scientists could analyze and publish the data in peer reviewed journals. But there is no way to access the data and even FOIA requests on post-marketing surveillance studies are not addressed in a timely way. To me the FDA’s unwillingness to make their data publicly available is proof the top priority of the FDA is not that the public thrives but that the product thrive.
Lack of response from the FDA regarding the process 2 DNA contamination of the Pfizer and Moderna COVID-19 vaccines, is another example of the agency’s lack of willingness to communicate with the public and citizen scientists on important issues. My friends and colleagues Phil Buckhaults, PhD and Kevin McKernan, PhD have sounded the alarm about a potential danger- that is because the billions of plasmid DNA particles are encapsulated in LNPs they will be better at transfecting the cell and migrating to the nucleus. The Pfizer vaccine in particular has the SV40 promoter and enhancer in the plasmid genome which will increase (at least to my knowledge) the chance of DNA integration. The questions to the FDA are 1. when did they learn about this potential problem, 2. have the checked for integration into the human germline, 3. if not are they planning to, 4. When did they learn about the SV 40 promoter in Pfizer and 5. why was the public not made aware (given informed consent) about the DNA contamination. More on our podcast here, now with 340k views!)
What the heck constitutes an “emergency”?
Take all of the currently available COVID-19 vaccines. All of the current formulations are authorized, even the pediatric ones, under “emergency use”. Dare I say if everything is an emergency then nothing is. Rather than having one person proclaim an “emergency” it seems like we need to have some sort of minimum standard, set definition or public process for determining whether something truly warrants emergency authorisation. To what extent the authorisation of the COVID-19 vaccines was “militarized” due to the PREP act is still something I am trying to fully understand. But I do suspect it relates to the next point which is that none of the side effects or risks of the covid vaccines are disclosed in advertisements. Why?
Direct-to-consumer advertising.
On the surface it may seem like a nothingburger. Why should the pharmaceutical industry not be allowed to advertise like every other industry? But then maybe we should consider why it is the US and New Zealand are the only countries that have not outlawed direct to consumer pharmaceutical advertising. Having lived and practiced medicine in two countries with different approaches, I want to mention a couple of considerations I think are important:
The media in the US is beholden to the phamaceutical industry. They will not write pieces that investigate corruption in the FDA or the industry if the industry is the main or even major source of their paycheck. Same goes for academics, especially those with NIH funding. Our press and academic institutions have become inherently conflicted due to the fact that the pharmaceutical industry advertisements directly or indirectly pay the salaries of so many influential people in this country. Denmark has also outlawed pharmaceutical adverts in its country’s medical journal (Ugeskrift for Læger)
Americans (from the US) compared with Danes are to a much greater extent under the perception that health is something you get in a bottle, an injection, a pill, through a surgery or at the hospital (you know, a “Center of Excellence”🙄). It’s all over the tv, billboards, in grocery stores, the print media. We can’t escape this mentality. In Denmark, health is spelled with a lower case h. It is something you get through the way you live, the fun you have with people you love, the exercise you do, the way you travel to work and school, the time you spend outdoors, the way you eat and sleep. The more we focus on capital H Health in the US, the less healthy we as a society become.
The less wealthy, too. I’m sure I am not the only doctor reading this who listens to patient after patient describe how they can’t afford their insurance premiums and co-pays. Co-pays for basic things like physical therapy.
I’m sure I’ve missed a lot. Please help me add to the list of FDA shortcomings.
In the meantime, I want to thank Bret Weinstein and Rav Arora for their recent podcast, which partially inspired this post- they reminded me that I should stop being such a nihilist, believing the FDA can’t be salvaged, because honestly, although I do think public distrust is healthy, we need our regulators and public health officials.
Also, I seriously can’t get enough of this song right now. Basic beatbox tune but dang, Diljit Dosanjh has in incredible voice. I think they are singing in Punjabi.. but what are they saying?