The Child Immunization Schedule in the US vs Denmark: Why is there such a big difference & how can we use the next four years to improve our evidence base?
Substack Post & Vaccine Curious Podcast by Christine Stabell Benn, MD, PhD & Tracy Beth Høeg, MD, PhD
With the new presidential administration in the US, more focus has been brought to the fact that the United States’ approach to public health - including their recommended vaccination schedule - differ substantially from Europe’s.
We have done a series of podcasts over the last year and a half, which have brought specific attention to the way the US childhood vaccination schedule differs from that in Denmark. We have discussed the data and why the two countries may have made different decisions. One thing that quickly became clear to us is that there is both an opportunity and a need for better research into vaccination recommendations as well as overall approaches to healthy living. In other words, we need a better understanding of what truly leads to better health.
We are both Danish MD, PhD physicians, vaccine researchers and mothers; Christine lives in Denmark and studies vaccines at University of Southern Denmark. Tracy Beth lives in the US and studies vaccine safety at MIT.
In this podcast, which we recorded today and article below it, we summarize the insights we gained from our discussions:
Here is a version you can watch on Substack:
This version will take you to a link where you can download the video
Also available on Spotify
& Apple.
Our key messages are:
We need a system of public health that focuses on improving overall health, rather than preventing specific diseases (we will discuss how Denmark has been more successful at this than the US)
This also applies to vaccines for the following reasons:
Vaccines have been shown in numerous randomized controlled trials, primarily in low-income settings, to have non-specific health effects. Live attenuated vaccines have been shown to harness beneficial non-specific effects (leading to reduced risk of non-target diseases or overall mortality). However, non-live vaccines have been observed to be associated with negative non-specific effects (increased risk of non-target infections and mortality, primarily in females). The effects are seen as long as a given vaccine is the most recent, and can be reversed with a new type of vaccine.
Vaccines can cause serious adverse events such as cardiovascular effects (e.g. myocarditis, disorder of blood clotting) and neurological effects (e.g. narcolepsy, Bell’s Palsy, transverse myelitis) as well as complex multi-organ disease (e.g.post-acute COVID vaccine syndrome).
The current system for testing, approving and monitoring vaccines is not set up to capture non-specific effects and has serious limitations when it comes to capturing adverse events. Thus, studies of vaccines should aim to look at overall health impacts and not just the impact on the target disease. This is particularly important when the risk of the target disease is low.
Introduction
The current paradigm for health
In the most recent definition of health, from the WHO, health is “a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity”. Many health systems, however, have mainly focused on treating specific diseases, rather than strengthening people’s health.
Challenges with the current framework for health
A focus on treating illnesses means that prevention is not prioritized, even though a focus on making people healthy - e.g. through support for exercise, healthy diet and living, and interventions that strengthen people’s general health and resilience - would increase quality of life and be highly cost-effective.
In Denmark, health is more associated with living well through time in nature, cycling or walking to work and school. Maternity and paternity leave is seen as a type of health intervention. All health care is free of charge. This helps keep healthcare costs down. No one in Denmark goes bankrupt from medical bills whereas in the US medical bills cause ⅔ of all bankruptcies.
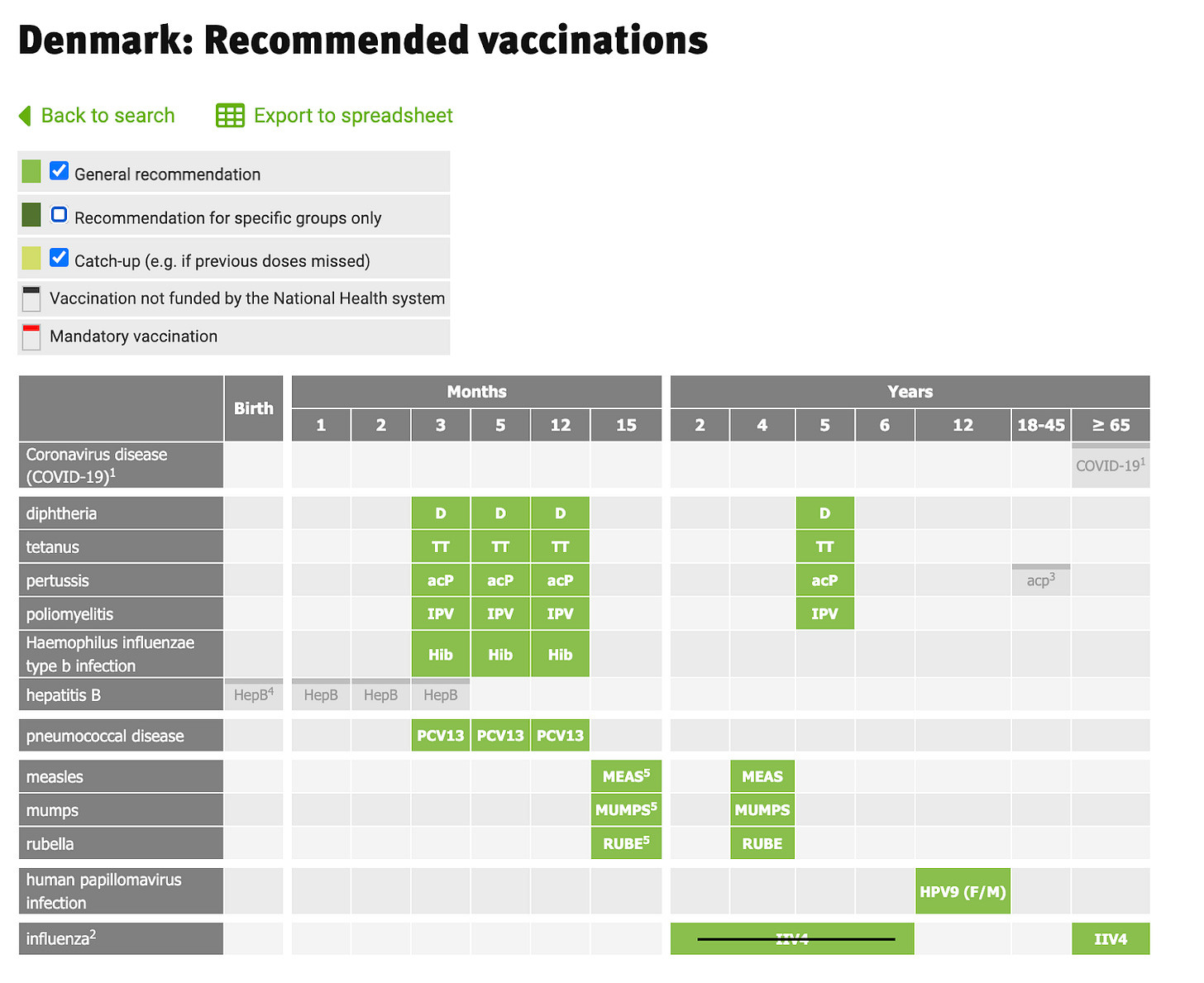
In the US, health is more often seen as something that is achieved through medical treatments, interventions, surgeries, etc. More than Denmark, the US associates “doing more” medically with “good medicine”, which may explain why the US has almost 4x as many doses of vaccines recommended for children than Denmark; Denmark vaccinates against 10 diseases and the US 18. Denmark does not routinely vaccinate children against COVID-19, influenza, rotavirus, RSV, varicella (chickenpox), meningococci, or hepatitis A/B. Some of the differences may originate from differences in underlying health risk of the average child in the US, but some may also stem from direct-to-consumer advertising and pharmaceutical influence on the CDC and FDA.
Interestingly, in 2021, Denmark started recommending the influenza vaccine to children 2-6 years, but this year they stopped the recommendation, citing low uptake as the reason - as the primary goal of vaccinating this age group was to reduce community spread of the flu, to protect the elderly.
The current paradigm for vaccines
The current paradigm for vaccines - that a vaccine is a biological preparation which induces specific immunity to a specific infection - dictates how vaccines are tested, regulated, approved, and monitored. This is done by assessing the vaccine effect on the specific infection (sometimes just on the induced antibody towards the specific infection). In phase 3 trials, plausible potential adverse events in the weeks following vaccination are recorded, as are serious adverse events such as disease leading to hospitalization and deaths during the short follow-up. After a vaccine has been approved, it is seldom evaluated in trials. Most post-licensure evaluation is based on doctors and citizens reporting potential adverse events linked to vaccination (so-called “passive surveillance”).
Challenges with the current framework for vaccines
The current framework has clear limitations when it comes to detecting rare but serious adverse events as well as small increases in common serious events. They are either too rare or deviate too little from the baseline rate to be significant and thus both can fail to be captured in phase 3 trials. Following licensure, there is a well known underreporting of adverse events during what is often termed “post-marketing surveillance”. New/relatively rare diseases occurring shortly after vaccination, such as myocarditis in young men post-COVID-19 vaccination, may be captured. However, adverse events that occur relatively often such as heart attacks or strokes in elderly people, may be very difficult to capture. This is particularly the case for adverse events that manifest as multi-organ disease, where the dominating symptom can imply that it is reported e.g. by one MD as a neurological disease, but by another as a gastrointestinal disease. This makes it difficult to trace back to the vaccine and to establish a pattern. However, even a small increase in myocardial infarction, miscarriage, hospitalization, or death rates due to the vaccine would be devastating, but difficult to detect statistically, if not evaluated in large-scale randomized trials.
Importantly, the current framework was developed before it was known that vaccines can affect the general immune system and the risk of other diseases more broadly. It is now clear from epidemiological and immunological research that this understanding of vaccines is too narrow. It follows that one cannot be sure that just because a vaccine protects against the specific infection that it will be beneficial for the overall health. The best known example is the high-titre measles vaccine that was fully protective against measles, but nonetheless was associated with 2-fold statistically significantly higher all-cause mortality in females, which led to its withdrawal.
The fact that the currently used vaccines were not tested for their overall health effect now puts health authorities globally in the problematic position that when vaccine hesitancy is increasing, they do not have the necessary studies and data to prove that one becomes overall healthier by getting vaccinated than not. This means that vaccine hesitancy will likely continue to increase until adequate studies have been performed, and for each and every vaccine - both those already in use and those under development - there is clear and unequivocal data that shows that the vaccine is overall beneficial.
Vaccination schedule differences between US and Denmark
Few Americans are aware that Denmark does not offer yearly COVID-19 or influenza vaccine to non-high-risk children or adults under the age of 65, nor the hepatitis B vaccine at birth or during childhood unless a child is at high risk (for example is born to a hepatitis B positive mother), nor the rotavirus or chickenpox vaccines or many other vaccines considered relevant or even mandated in the US, including 2 doses of varicella (chickenpox) and 3 doses of hepatitis B vaccines, which are mandated in states like California but not even offered to children in Denmark. Denmark only vaccinates for a total of 10 diseases in childhood, and only those that can cause severe disease in the child. In contrast, the US vaccinates for 18 diseases with 68 doses. See Tracy’s post about this on X here.
The view of the Danish Health Authorities is that it is best not to recommend too many vaccines, as this would likely increase vaccine hesitancy. The vaccines that are not offered by the government can, however, be purchased privately.This includes vaccines against hepatitis A/B, influenza, RSV, meningitis, and chickenpox. In order to obtain a free COVID-19 vaccine under the age of 65 in Denmark, a person must have one or more chronic conditions documented by a physician. Some vaccines which are not on the child vaccination schedule in Denmark can be ordered and paid for if parents wish to purchase them. These include influenza, chickenpox, hepatitis A & B, rotavirus, and tuberculosis.
The Danish vaccination schedule for children and adults
The US vaccination schedule for children
The US vaccination schedule for adults
The above Table was created by Jonaton Palleson reds out the childhood immunizations the US gives but Denmark does not
In general, vaccination is viewed differently in Denmark. Historically, Denmark has only recommended vaccines for diseases that caused severe disease in the child. There are no mandates for children to receive vaccines in order to attend school. The COVID-19 era saw a bit of a departure from this in that children down to the age of 5 years were recommended COVID-19 vaccines. However, this practice was quickly dropped and in the summer of 2022, the head of the Danish Health Authority declared that “In retrospect, we didn't gain much from expanding the vaccination program for children when it comes to epidemic control”.
European countries have been in general more hesitant to vaccinate children for low risk diseases. This may have been exacerbated by Europe’s experience with the Pandemrix influenza vaccine given to prevent H1N1 in 2009. Likely hundreds of cases of narcolepsy developed in children due to this vaccination for a disease which was in general low risk to them.
Our wishes for the coming four years
Our wishes for the coming period can be summarized in seven points:
A revised framework for testing, approving and regulating new vaccines
We would like to see a revised framework for testing, approving and regulating vaccines. The main changes to the current system would be that phase 3 trials should capture any new disease, in particular any new infection, arising after vaccination, not just those related to the vaccine disease and the most severe adverse events. E.g., a respiratory infection with Streptococcus pneumonia 3 months post-vaccination would currently not be captured in a phase 3 trial of the COVID-19 vaccines unless it led to hospitalization or death, but it could be a result of vaccination. Evidence of vaccines altering our risks of non-target diseases have been found in numerous observational studies and dozens of randomized studies, and it is no longer tenable not to assess them.
For example, this was not only seen with the COVID-19 mRNA vaccines in infants and toddlers but with the inactivated influenza vaccine, which was found in an RCT to increase risk of non-influenza respiratory infections by over 4 fold in children 6-15 years old.
A revision of the current protocol for phase 3 trials should go hand in hand with extensive phase 4 trials, where randomization or step-wedged roll out of vaccines should ensure that there would be similar large groups of vaccinated and unvaccinated individuals to be followed for overall health outcomes (all-cause mortality and hospitalizations, GP visits etc) for at least 2 years, allowing for comparison of their overall health.
A recent example of a vaccine that has been approved but where there in our view are safety signals that have not been sufficiently addressed: A randomized trial of the RSV vaccine to be given during pregnancy found a significant efficacy against RSV lower respiratory tract infection, but not against all cause lower respiratory tract infection and a greater than 20% but non significant rise in preterm birth. Thus it was clear the trial was underpowered to determine critical health consequences but the vaccine was nonetheless approved. During the approval process it was stated adverse events would be monitored in post-licensure surveillance, but this type of monitoring is slow and will be biased by underlying differences in health in vaccinated vs unvaccinated mothers.
Many vaccines are recommended to healthcare workers and/or the general population based on the belief they will reduce transmission to others. However, to date, this has not been proven for vaccinations as old as the influenza vaccine. Vaccines that are intended to be used to reduce transmission to others should have transmission to household members (or contacts for example in a healthcare setting, etc) tested to document that transmission rates are indeed reduced.
Health authorities that carefully reviews all safety signals in relation to vaccines
In our podcasts we among others discussed some of the safety concerns in relation to COVID-19 vaccines. They included a discussion of the switch in production of the mRNA vaccine and the problem of residual DNA fragments in the vaccine batches produced with the new method. These safety issues have been dismissed by FDA and other regulatory bodies. We would like to see regulatory bodies take much more swift action when such issues arise. It is not reassuring to just reply that there is no reason for concern, if public trust is to be maintained. This was also seen in the US when post-vaccination myocarditis among adolescents and young adults was downplayed and not addressed properly by the CDC. For example, an alert that had been written by the CDC about post-vaccination myocarditis was not released due to unknown reasons in the Spring of 2021. After 14 post-vaccination cases had been reported in the US military, the US CDC director maintained that no link had been seen between the vaccination and myocarditis in the US.
Stopping unnecessary vaccines
As outlined above, there are major differences in the US and the Danish vaccination program and the question which follows is of course if all the US vaccines are necessary.
One question of relevance to both countries is the recommendation to receive two doses of HPV vaccine. WHO has declared that one dose of HPV vaccine is enough, and the logical implication would be to change the recommendation accordingly.
Another important question is whether a hepatitis B vaccine at birth is necessary. The US is vaccinating all children at birth to prevent the few mothers who are hepatitis B positive from transmitting the virus to their children. This means that >95% of those vaccinated at birth do not need the vaccine at birth. Denmark (and almost all of Europe) does not recommend universal hepatitis B vaccine; pregnant women are screened for hepatitis B in pregnancy and only children of positive mothers are vaccinated. Hepatitis B vaccine is also not part of the childhood vaccination program; one can eventually buy the vaccine later in life, if at high risk of exposure, e.g. working with blood products.
The overall health effects of hepatitis B vaccine have never been studied. Two studies from Africa, from Guinea-Bissau and The Gambia, have indicated that the vaccine may have negative non-specific effects in females, like it has been seen for other non-live vaccines. In our view, there is no reason for the US to provide universal hepatitis B vaccine at birth, since the US also screens pregnant women, and can thus target hepatitis B vaccination to infants at risk.
Constructive research on the vaccines already in use
If it was felt to be too drastic to stop hepatitis B vaccine immediately, it would be possible to test the hepatitis B policy, by randomly allocating children to receive the currently recommended schedule, or to receive a schedule with no hepatitis B vaccine, and then assess the overall health effect (such as all-cause t mortality, all-cause hospitalisation rate, infectious disease hospitalisation rate, self-reported infections, autism, ADHD, etc...) up to 1) the age of the next planned vaccines; 2) age 2-3 years; 3) age 10 years. One could then possibly offer the hepatitis B vaccine at the time of HPV vaccine to those that did not get it in childhood.
Also, research into the immune training effects of life and non-live vaccines have indicated that it is better to have a live vaccine as the most recent vaccine. E.g., a study of >300,000 US children showed lower risk of non-targeted disease hospitalizations from age 16 through 24 months among children whose last vaccine received was live compared with non-live vaccine, as well as concurrent receipt compared with non-live vaccine. It would be interesting to test the live attenuated and the non-live injected influenza vaccine against each other in a randomized trial. Furthermore important would be to test whether modifications of the current program, emphasising having a “live vaccine last” would reduce all-cause morbidity. Based on a WHO review, it was calculated that such a schedule has the potential to reduce all-cause child mortality by 1 mill deaths/year.
Individual case reports have linked the measles-mumps-rubella (MMR) vaccine to autism. Danish register-based observational studies of the policy of providing MMR as a single vaccine at age 15 months found no link between MMR and autism risk. In the US, MMR is given with non-live vaccines. Epidemiological studies have shown that live vaccines alone vs. combination of live and non-live vaccines have different mortality and morbidity effects. It would be interesting to test in the US if MMR with other vaccines have similar effects as MMR alone.
No vaccine mandates
UNESCO’s ”Universal Declaration on Bioethics and Human Rights” article 6.1. states that ”Any preventive medical intervention is only to be carried out with the prior, free and informed consent of the person concerned, based on adequate information”. While not legally binding, the meaning is clear: vaccines should not be mandatory. Denmark adheres to this principle and still has very high vaccination coverage of >90% for all childhood vaccines except for HPV vaccine.
It should be a human right to weigh pros, cons and unknowns to make one’s own informed decision. Promoting forced vaccination may increase resistance. Those who care for public health should promote vaccines with data showing that it protects against e.g., measles and improves overall health. Adherence to vaccine recommendations is built with good data showing clear overall health benefits of being vaccinated, and with transparency about what is known and what is still not known.
A more open and transparent discussion of the pros and cons of vaccines
In the US, there is a culture of demonizing people in healthcare for questioning the utility or safety of vaccines, even when they are vaccines not given routinely in European countries.
In a recent experiment, conducted on large, representative samples of Americans and Danes, the effect of vague vaccine communication was contrasted with that of transparent communication, which disclosed either positive or negative vaccine features. The study showed that vague, reassuring communication had negative effects on acceptance by eroding trust in health authorities and increasing the reception of conspiracy theories. To deserve and maintain public trust it is thus essential that health authorities communicate both the pros and cons of vaccines, along with the knowns and unknowns.
A general focus on overall health
Last, but not least, we endorse a general focus on overall health - a focus that applies far beyond the area of vaccines.
As pointed out by Prasad and Cifu, there is a long list of so-called “medical reversals”. These are situations where health professionals wrongly presumed that because an intervention had an effect on an intermediate outcome, like a biomarker, or because a screening program captures a cancer earlier than it would normally have been diagnosed, then it would improve overall health. A focus on assessing the overall health effects of interventions or preventive programs is likely to prevent many medical reversals in the future.
But more importantly, a health system that focuses on strengthening overall health in people to prevent disease rather than treating their diseases once they do become ill, is likely to increase quality of life and reduce health care costs.
Conclusion
We are looking into a new period in American health history with optimism. Right now, there is a unique opportunity to improve health and to provide much-needed information to parents weighing anticipated risks and benefits of vaccination on overall child health.
Thank you to Frederik Schaltz-Bucholzer, MD, PhD for his very helpful fact checking and review of this post.